H.C. Wainwright has maintained a Buy rating on Kazia Therapeutics and left its price objective at $18.00 following newly disclosed clinical data from the company’s Phase 1b study of paxalisib. The update, presented on January 27, covers the combination of paxalisib with pembrolizumab and chemotherapy for patients with late-stage, metastatic triple-negative breast cancer (TNBC).
The company’s stock is currently priced at $7.15 and carries a market capitalization of $77.91 million. H.C. Wainwright also reiterated the stock’s higher volatility profile, reflected in a reported beta of 1.75, consistent with the risk characteristics common to early-stage biotechnology firms.
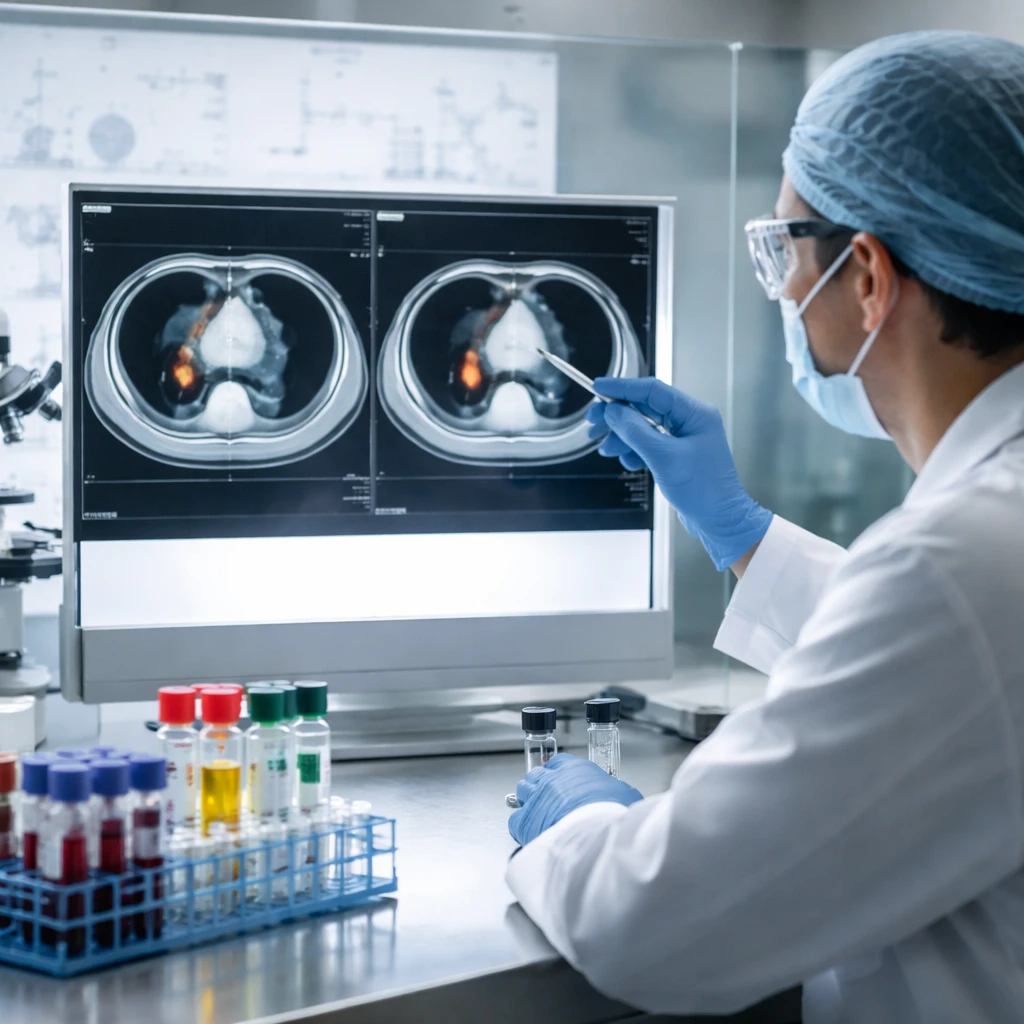
According to the clinical update, both evaluable patients treated at the 30 mg paxalisib dose experienced partial responses after receiving at least three cycles of therapy. These outcomes build on earlier information disclosed in December 2025, when the first treated patient registered 76% tumor shrinkage.
An additional individual who received the combination regimen through an expanded access pathway - and who had been ineligible for trial enrollment due to prior pembrolizumab exposure - achieved a confirmed complete metabolic response. The results were described by H.C. Wainwright as "very encouraging," particularly when compared with historical response rates for pembrolizumab plus chemotherapy, which the firm notes can reach, at best, approximately 53% depending on PD-L1 expression status.
Investors tracking analyst expectations will find a range of price targets reported via InvestingPro, spanning $18.90 to $21.08. The firm also flagged that Kazia has not been profitable over the last twelve months and rated the company’s financial health as FAIR within the InvestingPro framework, with more detailed metrics and ProTips available to subscribers.
Further context from Kazia’s recent disclosures underscores ongoing clinical and corporate developments. In its Phase 1b program, three patients with metastatic TNBC showed clinically meaningful responses to the paxalisib plus pembrolizumab and chemotherapy regimen. One of those patients demonstrated a 76% reduction in tumor volume, with computed tomography imaging showing a fall from 154 mm2 to 36 mm2.
On the corporate-compliance front, Kazia announced it has regained full compliance with Nasdaq listing requirements after meeting the $2.5 million minimum stockholders’ equity threshold. That restoration of compliance resulted in the cancellation of a previously scheduled hearing before the Nasdaq Hearings Panel.
H.C. Wainwright’s decision to raise its price target from $13 to $18 - while maintaining a Buy recommendation - followed the updated breast cancer trial data presented at the 2025 San Antonio Breast Cancer Symposium. Collectively, these clinical readouts and the company’s return to Nasdaq compliance have prompted the analyst community to reassess near-term expectations for the stock.
While the clinical results reported to date are limited in scale, they represent positive signals within the confines of an early-phase study. The market reaction and analyst coverage reflect both the potential upside implied by the new data and the typical uncertainties tied to small-cap, early-stage biotechnology companies.
Sector impact: Biotech and oncology-focused healthcare equities are the primary sectors affected by this update, with potential spillover interest among small-cap and Nasdaq-listed stocks.